European Cancer Summit: Keynote speech by EU Commissioner Stella Kyriakides
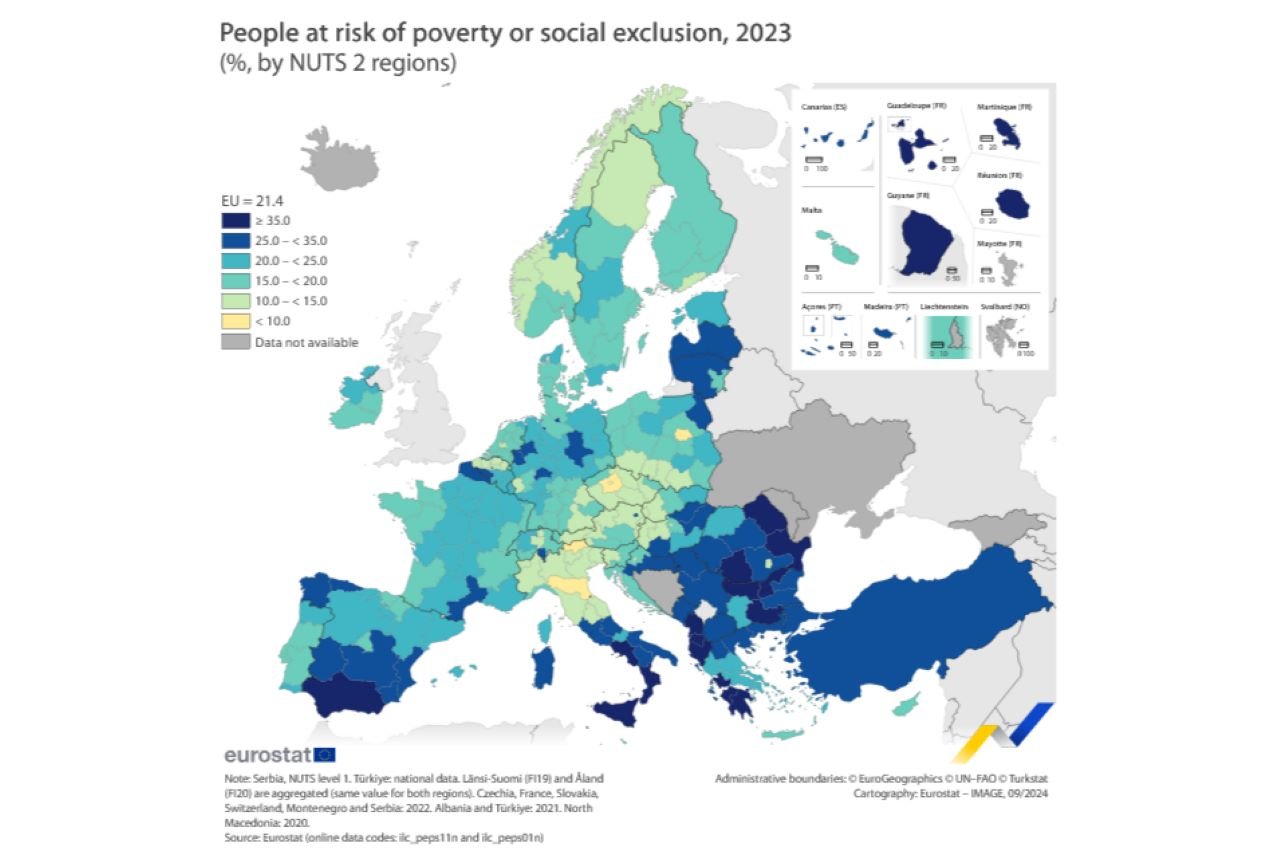
“Member States and stakeholders are now also working together to improve and extend cancer screening programmes across the EU with the new updated recommendations on cancer screening and funding under the EU4Health Programme, but we still have huge inequalities in access to screening across the EU” – EU Commissioner Kyriakides.