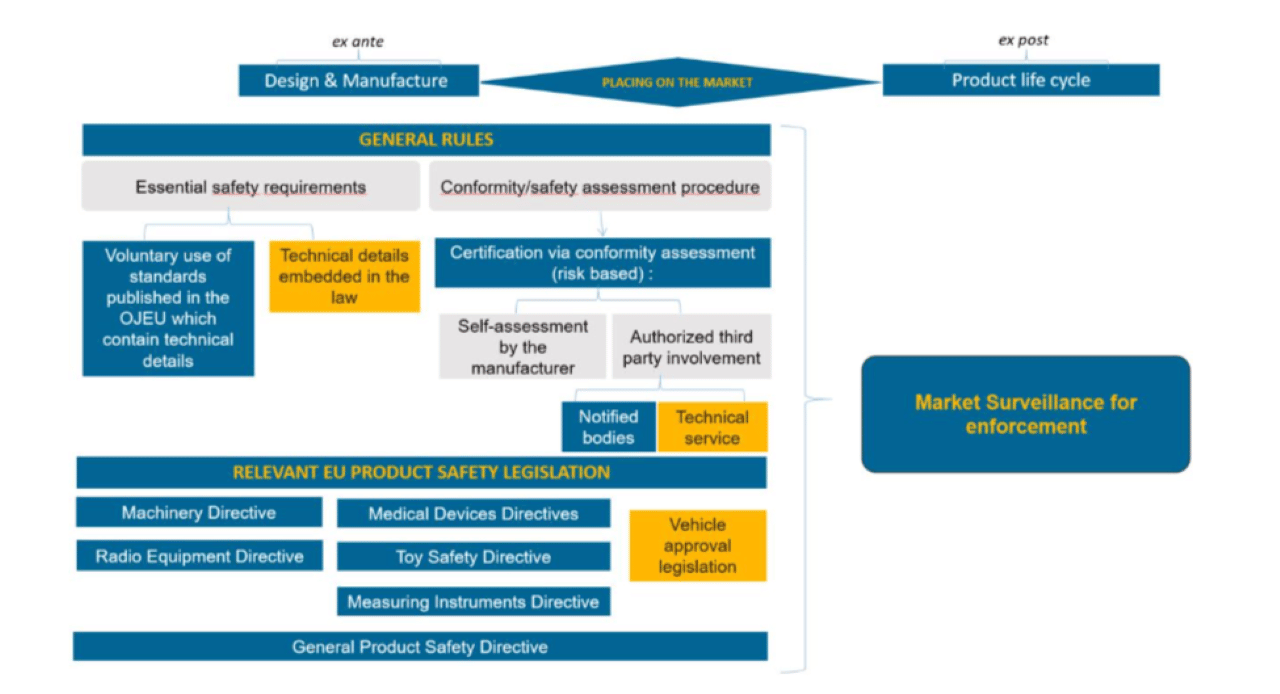
PRODUCT SAFETY
- EU Commission welcomes political agreement on the new EU product safety rules
- Products imported from third countries into the EU will be safer thanks to better controls ensured by S&Ds
- René Repasi (Europa-SPD) zur Produktsicherheit: Verbraucher-Rechte gegen gefährliche Waren stärken
- Marion Walsmann (CDU) zur Trilog-Einigung bei Produktsicherheit
BIODIVERSITY
- COP15: the European Union and the Organisation of African, Caribbean, and Pacific States call for an ambitious global agreement
SUSTAINABILITY
- The Partnership between the U.S. and Russia in the Field of Sustainable Development Goals in the Arctic Region
CIRCULAR ECONOMY
- Non-transposition of EU legislation: Commission takes action to ensure complete and timely transposition of EU directives
INDUSTRIAL DESIGN
- Intellectual property: New rules will make industrial designs quicker, cheaper and more predictable
BIOPLASTICS
- Draft revision proposal of PPWD: bioplastics industry sends incendiary letter to Commission Presidency regarding devastating consequences for the sector
ECHA
- Rodent traps can be effective at controlling house mice infestations
ANTITRUST
- Antitrust: Commission fines styrene purchasers €157 million in cartel settlement
WATER RESOURCES
- State of Global Water Resources report informs on rivers, land water storage and glaciers – World Meteorological Organization
EEA
- Natura 2000 data – the European network of protected sites
EU PARLIAMENT REGISTER
- DRAFT MOTION FOR A RESOLUTION on the draft Commission implementing decision granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of the Council for “Comirnaty – tozinameran, COVID-19 mRNA vaccine (nucleoside-modified)”, a medicinal product for human use and repealing Decision C(2020) 9598(final) – PE738.499v01-00
- DRAFT MOTION FOR A RESOLUTION on the draft Commission implementing decision granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of the Council for “Spikevax – elasomeran”, a medicinal product for human use and repealing Decision C(2021) 94(final) – PE738.571v01-00
- DRAFT MOTION FOR A RESOLUTION on the draft Commission implementing decision amending the marketing authorisation granted by Decision C(2022) 7342(final) for “Comirnaty – tozinameran, COVID-19 mRNA vaccine (nucleoside-modified)”, a medicinal product for human use – PE738.722v01-00
- DRAFT MOTION FOR A RESOLUTION on the draft Commission Implementing Decision renewing the authorisation for the placing on the market of products containing, consisting of or produced from genetically modified soybean A5547-127 (ACS-GMØØ6-4) pursuant to Regulation (EC) No 1829/2003 of the European Parliament and of the Council – PE738.497v01-00
- DRAFT MOTION FOR A RESOLUTION on the draft Commission implementing regulation granting a Union authorisation for the biocidal product family ‘CMIT/MIT SOLVENT BASED’ in accordance with Regulation (EU) No 528/2012 of the European Parliament and of the Council – PE738.498v01-00
- Draft agenda – Tuesday, 29 November 2022 – Thursday, 1 December 2022 – PE739.500v01-00 – Committee on the Environment, Public Health and Food Safety
SCHEER BRIEFS
- SCHEER – Minutes of the WG on Draft Environmental Quality Standards for the Water Framework Directive Priority Substances & groundwater quality standards of 18 November 2
- SCHEER – Minutes of the Working Group meeting on Cobalt in toys of 21 November 2022
You can buy and download this issue as a PDF:
Important note
On this platform, we are publishing our Insight EU Monitoring editions with a delay of several hours. If you want to get our monitoring e-mails in real-time you can subscribe to our full-text services on our Insight EU Store page.
Please, do not hesitate to reach out to Insight EU if you need tailored regulatory monitoring services, focusing on certain policy fields, institutions, or issues.
- See our FAQs for further information.
- Subscribe to our Insight EU Premium offer: portal.ieu-monitoring.com/insight-eu-premium/
- Free Daily Insight EU Digest Basic: portal.ieu-monitoring.com/ieu-daily-digest/
- Weekly Insight EU Agenda: portal.ieu-monitoring.com/ieu-agenda/
- Specialized Insight EU monitoring issues: portal.ieu-monitoring.com/ieu-store/
- Ask our team for a tailored and targeted offer: sales[at]ieu-monitoring.com.