EP ENVI COMMITTEE
- Meeting of the Committee on Environment, Public Health and Food Safety (ENVI)
ECHA
- New ECHA Public Data Availability System – Part II
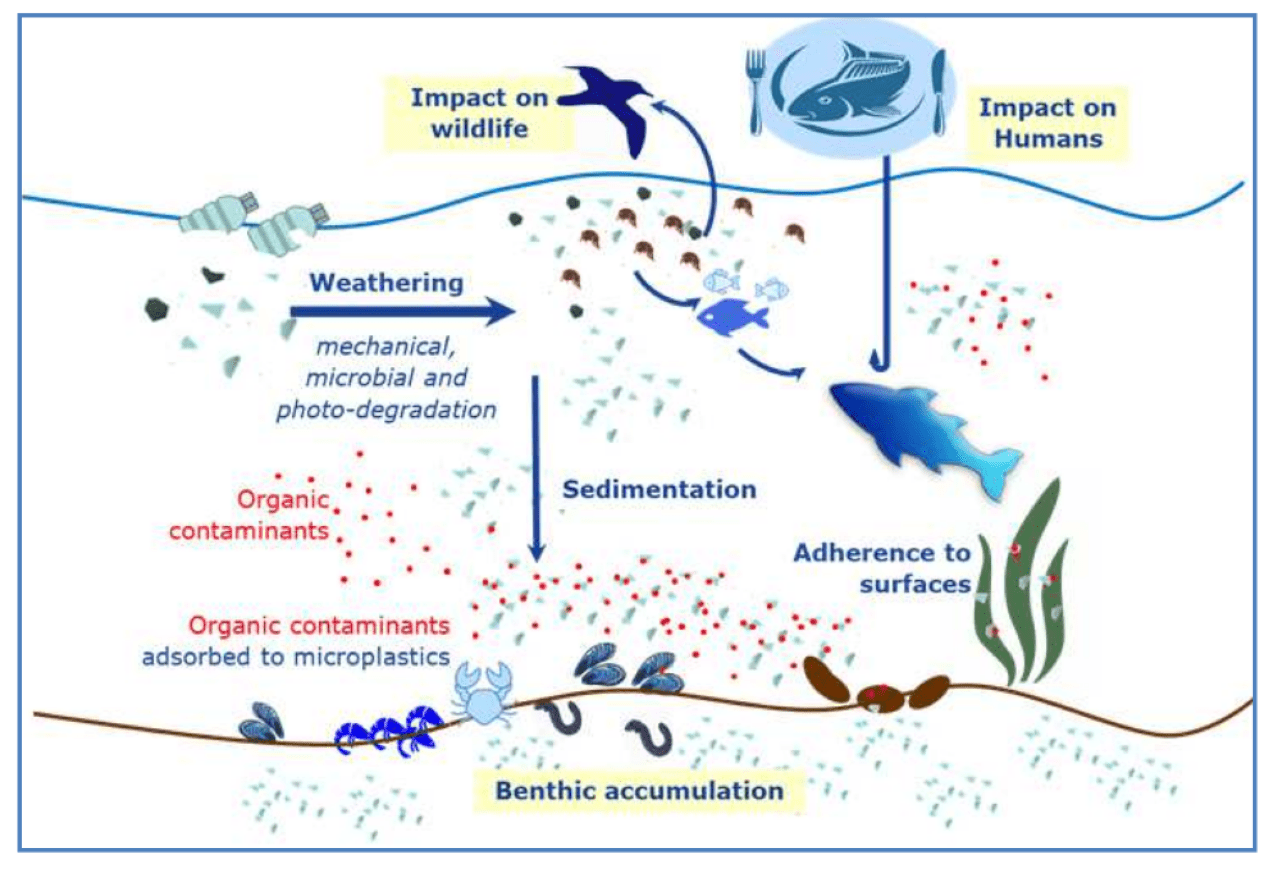
PLASTICS
- Plastics Europe launches Plastics – the fast Facts 2023
PROTEIN STRATEGY
- European Protein Strategy: EU must increase protein production and diversify supply chains
ANIMAL WELFARE
- Eurobarometer shows how important Animal Welfare is for Europeans
PACKAGING WASTE
- Eurostat: EU packaging waste generation with record increase
UKRAINE
- Commissioner Sinkevičius in Ukraine to discuss environmental damage to the country
GERMANY
- Konferenz Zukunft gestalten – Transformation, gemeinsam, jetzt: Nachhaltigkeit gelingt nur, wenn alle mitmachen
- Abgesetzt von der Tagesordnung des Bundestags: Exportverbot für nicht zugelassene Pflanzenschutzmittel
- EU-Verpackungsverordnung: CDU/CSU für weniger Bürokratie
SCHEER BRIEFS
- SCHEER – Minutes of the 6th plenary meeting, Luxembourg, 6 October 2023
EFSA BRIEFS
- Assessment of the feed additive consisting of niacinamide for all animal species for the renewal of its authorisation (Arxada Ltd)Assessment of the probability of introduction of Thaumatotibia leucotreta into the European Union with import of cut roses
- Safety and efficacy of a feed additive consisting of endo‐1,4‐beta‐xylanase (produced by Aspergillus oryzae DSM 33700) (RONOZYME® WX (CT/L)) for all poultry species and all Suidae (DSM nutritional products ltd)
- Safety and efficacy of a feed additive consisting of Enterococcus faecium DSM 33761, Pediococcus acidilactici DSM 33758, Bifidobacterium animalis DSM 16284, Limosilactobacillus reuteri DSM 33751 and Ligilactobacillus salivarius DSM 16351 (Biomin® C5) for chickens for fattening, chickens reared for laying, turkeys for fattening, turkeys reared for breeding and minor poultry species for fattening and reared for laying/breeding (Biomin GmbH)
- Ad Hoc meeting with industry representatives (GMO applicants)
EU COUNCIL REGISTER
- ST 14367 2023 INIT Regulation on the certification of carbon removals: Agricultural and forestry aspects – state of play – Information from the Presidency
- ST 14214 2023 INIT Attendance of third party at the Working Party on International Food and Agricultural Questions (FAO) on 3 November 2023 – Approval
- CM 4941 2023 INIT Informal videoconference of the members of the Working Party on International Environment Issues (UNECE and other regional issues) (morning only)
- CM 4907 2023 INIT Informal videoconference of the Working Party on the Environment
- CM 4953 2023 INIT – Working Party on Genetic Resources and Innovation in Agriculture (Seeds, Propagating and Planting Materials)
- CM 4905 2023 INIT – Working Party on the Environment
- CM 4942 2023 INIT – Working Party Meeting on Technical Harmonisation (Fertilisers)
- ST 14409 2023 INIT – Proposal for a Regulation of the European Parliament and of the Council on fluorinated greenhouse gases, amending Directive (EU) 2019/1937 and repealing Regulation (EU) No 517/2014 – Letter to the Chair of the European Parliament Committee on the Environment, Public Health and Food Safety (ENVI)
- ST 14411 2023 INIT – Proposal for a Regulation of the European Parliament and of the Council on substances that deplete the ozone layer and repealing Regulation (EC) No 1005/2009 – Letter to the Chair of the European Parliament Committee on the Environment, Public Health and Food Safety (ENVI)
- ST 13330 2023 ADD 1 – Regulation on new genomic techniques (NGT) – comments from Finland, Germany, Greece, Hungary, Ireland, Italy, Lithuania
- ST 13330 2023 ADD 2 – Regulation on new genomic techniques (NGT) – comments from Luxembourg, the Netherlands, Poland, Romania, Slovakia, Slovenia, Sweden
EU PARLIAMENT REGISTER
- Answer to a written question – Glyphosate authorisation – E-002552/2023(ASW)
- Answer to a written question – Epizootic hemorrhagic disease (EHD) in Spain – E-002532/2023(ASW)
- Video of a plenary debate – European Citizens’ Initiative ‘Fur Free Europe’ (debate) – Thursday, 19 October 2023 – 09:05 – Strasbourg
- AMENDMENTS 1 – 9 – Draft opinion EU Action Plan: protecting and restoring marine ecosystems for sustainable and resilient fisheries – PE753.465v01-00
- At a Glance – Research for PECH Committee – Workshop on the European Green Deal − Challenges and opportunities for EU fisheries and aquaculture − Part III: Food security aspects – PE 752.451 – Committee on Fisheries
- Answer to a written question – The Chinese Government’s export restrictions on gallium and germanium – E-002165/2023(ASW)
- Study – Research for PECH Committee – Workshop on the European Green Deal – Challenges and opportunities for EU fisheries and aquaculture –Part II: Marine biodiversity aspects – PE 747.265 – Committee on Fisheries
EU COMMISSION REGISTER
- COMMISSION IMPLEMENTING REGULATION (EU) /… granting a Union authorisation for the biocidal product family ‘HCl Disinfecting Toilet Bowl Cleaner’ in accordance with Regulation (EU) No 528/2012 of the European Parliament and of the Council
- REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL on the implementation of Regulation (EC) No 762/2008 of the European Parliament and of the Council of 9 July 2008 on the submission by Member States of statistics on Aquaculture and repealing Council Regulation (EC) No 788/96
- COMMISSION DELEGATED REGULATION (EU) /… amending Regulation (EC) No 1272/2008 as regards the harmonised classification and labelling of certain substances
- COMMISSION IMPLEMENTING REGULATION (EU) /… amending Regulation (EU) No 37/2010 as regards the classification of the substance ketoprofen with respect to its maximum residue limit in foodstuffs of animal origin
- COMMISSION IMPLEMENTING DECISION amending the marketing authorisation granted by Decision C(2022) 7342(final) for “Comirnaty – tozinameran, COVID-19 mRNA vaccine (nucleoside-modified)”, a medicinal product for human use
A PDF and DOC version of the complete Insight EU Monitoring edition can be purchased and downloaded below as soon as the product link becomes available:
Please note that there may be a delay of several hours in the availability of single monitoring downloads. To receive real-time monitoring emails, subscribe to our full-text services on the Insight EU Store page.
If you need tailored regulatory monitoring services that focus on specific policy areas, institutions, or topics, please contact Insight EU without hesitation at sales@ieu-monitoring.com or follow one of the links below:
- Insight EU Premium (complete daily portal posts – except Insight EU Monitoring mails):
portal.ieu-monitoring.com/plans/subscribe - Specialized Insight EU Monitoring mails – subscriptions or single editions::
portal.ieu-monitoring.com/ieu-store/ - Tailored and targeted Insight EU Monitoring offers: portal.ieu-monitoring.com/order-form
- FAQs for further information
- Free basic Insight EU Monitoring Digest and weekly Insight EU Agenda:
portal.ieu-monitoring.com/free-digest-and-agenda